Lessons from the Synthetic Chemist Nature
Abstract
Covering: 1981 to 2015
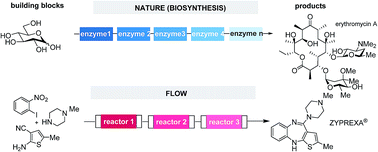
This conceptual review examines the ideal multistep synthesis from the perspective of nature. We suggest that besides step- and redox economies, one other key to efficiency is steady state processing with intermediates that are immediately transformed to the next intermediate when formed. We discuss four of nature's strategies (multicatalysis, domino reactions, iteration and compartmentation) that commonly proceed via short-lived intermediates and show that these strategies are also part of the chemist's portfolio. We particularly focus on compartmentation which in nature is found microscopically within cells (organelles) and between cells and on a molecular level on multiprotein scaffolds (e.g. in polyketide synthases) and demonstrate how compartmentation is manifested in modern multistep flow synthesis.

 Please wait while we load your content...
Please wait while we load your content...