Generation, structure and reactivity of tertiary organolithium reagents
Abstract
Covering: up to 2014
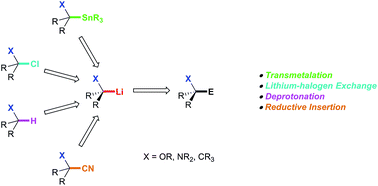
Tertiary alkyllithium reagents are very useful intermediates in synthesis. Alkyllithium reagents with adjacent heteroatoms may be formed stereoselectively or may react stereoselectively, and have been used in the synthesis of alkaloids, C-glycosides and spirocycles. An overview of the generation, reactivity and stereochemistry of tertiary alkyllithium reagents will be presented, as well as examples of their use in organic synthesis. The discussion will be focused on a conceptual understanding of the generation and reactivity of these intermediates. The reactions described herein generate fully substituted carbon atoms, and the forces driving stereoselectivity will be discussed in detail. Where appropriate, computational results will be introduced to provide a better understanding for the structure and reactivity of tertiary alkyllithium reagents.

 Please wait while we load your content...
Please wait while we load your content...