Catalytic intramolecular carbonyl–ene reaction with ketones: evidence for a retro–ene process†
Abstract
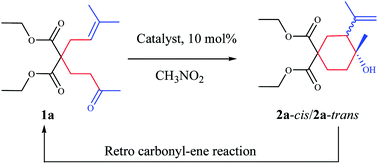
The ene-process with unsaturated ketones was catalyzed by Lewis acids such as bismuth or indium triflates. Unlike aldehydes, the reverse ene-process occurs with ketones, resulting in incomplete conversions, as shown by control experiments and analysis by ESI-MS.

 Please wait while we load your content...
Please wait while we load your content...