Dithionite and sulfinate complexes from the reaction of SO2 with decamethylsamarocene†
Abstract
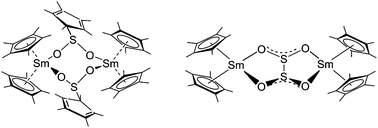
The reaction of [(η5-C5Me5)2Sm(THF)2] with SO2 resulted in four different products. The dimeric samarium complex [{(η5-C5Me5)2Sm(C5Me5SO2)}2] (1) crystallized from the reaction mixture first. The dithionite–sulfinate complex [{(η5-C5Me5)2Sm(S2O4)}2{(η5-C5Me5)Sm(C5Me5SO2)}2] (2), was obtained as the second major product after workup of the filtrate. Additionally, the dithionite complex [{(η5-C5Me5)2Sm}2(S2O4)] (3) and the sulfinate complex [{(η5-C5Me5)Sm}2(C5Me5SO2)4] (4) were isolated as minor products. All compounds were characterized by single crystal X-ray diffraction. As major reaction pathways, the reductive coupling of two SO2 molecules to form the dithionite anion S2O42− and the nucleophilic attack of one samarocene C5Me5 ligand on the sulfur atom of SO2 were observed.
- This article is part of the themed collection: Frontiers of Organo-f-Element Chemistry

 Please wait while we load your content...
Please wait while we load your content...