Photoinduced electron transfer from the oxo–MoIVselenolato complex to oxygen†
Abstract
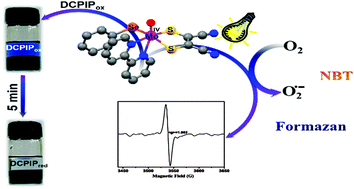
[NBu4][MoO(bpy)(mnt)(SePh)]·CH2Cl2 (1) and [NBu4][MoO(phen)(mnt)(SePh)]·CH2Cl2 (2) complexes; (bpy = 2,2′-bipyridine, phen = 1,10-phenanthroline, mnt = maleonitriledithiolate ion) stabilized by aromatic diimine have been synthesized and characterized. These complexes under aerobic conditions on irradiation with sunlight (or with light from a tungsten lamp) in solution generate superoxide radicals concomitant to the formation of paramagnetic Mo(V) species due to a single electron transfer from the Mo(IV) complex to oxygen. The Mo(V) produced under photo-irradiation was characterized by EPR spectroscopy and the generated superoxide radical has been shown to transform nitrobluetetrazoleum (NBT) chloride to blue diformazan. Both 1 (and 2) are phosphorescent having an emission lifetime of 6–8 μs. Ground state and time dependent density functional theoretical calculations confirm that the electronic transitions from predominantly metal based molecular orbitals are responsible for the low-lying charge transfer excited states and also for the observed interaction with oxygen.

 Please wait while we load your content...
Please wait while we load your content...