Tailoring the structure and electronic properties of platinum and gold–platinum nanocatalysts towards enhanced O2 activation†
Abstract
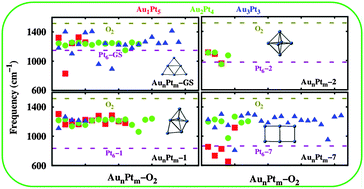
Geometric structure plays a crucial role in enhancing the catalytic activity of a material towards reactions such as oxygen reduction, methanol oxidation etc. Pt and Pt based bimetallic alloys of different morphologies such as nanorods, core–shell etc. have been synthesized so far contributing to varying catalytic activity in fuel cells. However, the electronic factors contributing to enhanced catalytic activity of particular structure/shape/morphology have not been understood so far. In a first such computational study, we demonstrate this complex structure–property correlation on model six-atom Pt clusters in the context of O2 activation at the cathode of direct methanol fuel cells. Since recent studies have shown that Au–Pt bimetallic alloys exhibit superior catalytic activity towards oxygen reduction reaction with respect to other bimetallic clusters, we have identified 4 distinct six-atom Pt cluster morphologies, viz., trigonal planar, distorted octahedral, normal octahedral and double square planar, and doped them sequentially with gold atoms until a ratio of 1 : 1 is obtained. Thus, clusters with varying conformations/shapes and bimetallic compositions are considered for potential O2 activation. The O–O bond stretching frequencies are calculated to evaluate the degree of bond activation in O2 molecules. Our study reveals that the Pt6 and AunPtm (n + m = 6) clusters with a double square planar shape exhibit superior catalytic activity towards O–O bond activation with respect to other six-atom conformations. Analysis of their electronic properties demonstrates that the amount of charge transferred by the cluster to the O2 molecule and the effective overlap between the d-orbitals of the metal atom and the pi-molecular orbitals of O2 can be directly correlated with their catalytic activities. In order to validate these conclusions, we have further extended such analysis to various Ptm (m = 3–13) conformations, wherein, once again the frontier molecular orbital composition is seen to play a predominant role in oxygen activation. Thus, the present study unveils the electronic properties determining the catalytic activity of a catalytic cluster towards O–O bond activation.

 Please wait while we load your content...
Please wait while we load your content...