Zinc(ii)-mediated generation of 5-amino substituted 2,3-dihydro-1,2,4-oxadiazoles and their further ZnII-catalyzed and O2-involving transformations†
Abstract
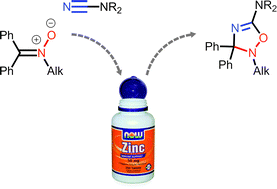
ZnII-activated cyanamides NCNR2 (R2 = Me2, Et2, C5H10, (CH2)2O(CH2)2, Ph2) react with the acyclic N-alkyl ketonitrones Ph2C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N+(O−)R′ (R′ = Me, CH2Ph) and N-aryl ketonitrones (R′ = Ph, p-BrC6H4, p-EtC6H4) under mild conditions. Uncomplexed 5-aminosubstituted 2,3-dihydro-1,2,4-oxadiazoles (6 examples; 49–82%) were obtained in zinc(II)-involving cycloaddition of the N-alkyl ketonitrones to the cyanamide substrates; these 2,3-dihydro-1,2,4-oxadiazoles undergo ring-opening giving carbamoylamidines and methylidenureas. The N-aryl ketonitrones react with ZnII-activated cyanamides giving the open-chain systems, viz. carbamoylamidines, N′-(2-(diphenylmethylidene)amino)-phenyl-N,N-carbamimidic acids, and methylidenureas, which are presumably formed via the cycloaddition route followed by the N–O cleavage induced by the acceptor character of the aryl groups.
N+(O−)R′ (R′ = Me, CH2Ph) and N-aryl ketonitrones (R′ = Ph, p-BrC6H4, p-EtC6H4) under mild conditions. Uncomplexed 5-aminosubstituted 2,3-dihydro-1,2,4-oxadiazoles (6 examples; 49–82%) were obtained in zinc(II)-involving cycloaddition of the N-alkyl ketonitrones to the cyanamide substrates; these 2,3-dihydro-1,2,4-oxadiazoles undergo ring-opening giving carbamoylamidines and methylidenureas. The N-aryl ketonitrones react with ZnII-activated cyanamides giving the open-chain systems, viz. carbamoylamidines, N′-(2-(diphenylmethylidene)amino)-phenyl-N,N-carbamimidic acids, and methylidenureas, which are presumably formed via the cycloaddition route followed by the N–O cleavage induced by the acceptor character of the aryl groups.

 Please wait while we load your content...
Please wait while we load your content...