Solvent effects on the nitrogen NMR chemical shifts in 1-methylazoles – a theoretical study†
Abstract
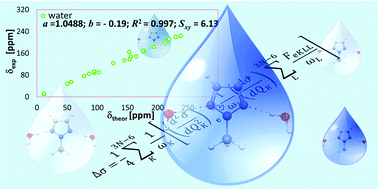
We have investigated solvent effect on the nitrogen chemical shifts in a series of 1-methylazoles. The detailed results for 1-methylazoles – systems containing one (1-methylpyrrole), two (diazoles), three (triazoles) and four (tetrazoles) nitrogen atoms in the heteroaromatic ring – have been presented. We have examined twenty six popular DFT functionals to calculate the nitrogen magnetic shielding constants in the gas phase and 12 different solvents within the conductor-like screening model (COSMO) and the explicit solvation model (ESM), as well as their combination (ESM + COSMO) in the case of water solutions. The vibrational corrections for the analyzed systems have been also reported. Additionally, the solvent effect on the nitrogen chemical shifts has been analyzed in terms of its direct and indirect contributions. Our theoretical vibrationally corrected results properly reproduce the experimental data. In the calculations of N NMR chemical shifts, the best results were achieved with the B97-2 functional with the mean absolute error as small as 3 ppm for a range exceeding 270 ppm in the tested azole systems.


 Please wait while we load your content...
Please wait while we load your content...