Conjugated aromatic asymmetrical terpyridine analogues via step-wise photocyclization and their ruthenium complexes†
Abstract
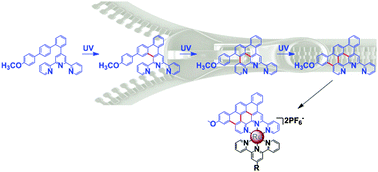
Novel terpyridine 1 was synthesized via Suzuki coupling; the resulting series of asymmetric conjugated terpyridine moieties were produced in good yield through subsequent UV irradiation. Oxidative cyclodehydrogenation was confirmed through isolation of the intermediate, proving the zippering cyclization in association with the aromatic conjugation expansion. The stepped conjugation could be controlled by choosing an appropriate solvent. The dimerization of terpyridine 1 was successfully introduced, making headway for these highly conjugated organometallic systems.

 Please wait while we load your content...
Please wait while we load your content...