Conversion of lapachol to lomatiol: synthesis of novel naphthoquinone derivatives†
Abstract
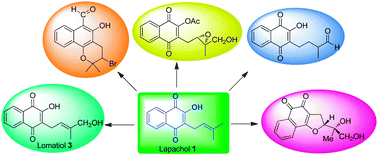
Lapachol (1), a naphthoquinone isolated mostly from the plants of the bignoniaceae family has a broad spectrum of biological activities and as a consequence it has been the object of different chemical transformations. Lomatiol (3), another naturally occurring naphthoquinone having structural similarities to lapachol, has been obtained from chemical and microbial transformations of lapachol in very low yields. In the present study, an easy approach for the synthesis of lomatiol (3) from lapachol (1) has been developed using SeO2 oxidation in 90% yield. Lomatiol, under epoxidation conditions afforded novel furano- and pyrano-naphthoquinone derivatives, which are analogues of anticancer agents, 2-acetylfuronaphthoquinone and β-lapachone. Most of the structures were unambiguously confirmed by single crystal X-ray analysis.

 Please wait while we load your content...
Please wait while we load your content...