Theoretically exploring the key role of the Lys412 residue in the conversion of N2O to N2 by nitrous oxide reductase from Achromobacter cycloclastes†
Abstract
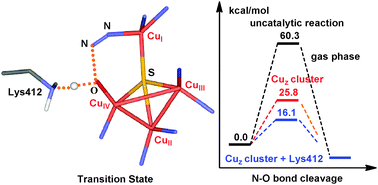
Density functional theory (DFT) calculations have been performed to investigate the N2O reduction reaction mechanism catalyzed by the μ4-sulfide-bridged tetranuclear CuZ cluster of nitrous oxide reductase from Achromobacter cycloclastes. Our calculations showed that the active site CuZ cluster can provide strong back-donation to N2O, facilitating N–O bond cleavage. In addition, the Lys412 residue near the CuI/CuIV edge of the CuZ cluster is considered as a suitable proton donor and plays a key role in the reduction reaction. The predicted activation barrier of N–O bond dissociation for the energetically favorable pathway with the terminal nitrogen atom of N2O coordinated to the CuI center is only 14.6 kcal mol−1 in the protein environment. The barrier is significantly lower than the barrier in the absence of the Lys412 residue and CuZ cluster. The relatively low barrier is ascribed to the N–O bond cleavage coupled with the oxygen-atom protonation of the coordinated N2O. Present calculations provide significant insights into the mode of substrate coordination, activation, reduction and catalysis.

 Please wait while we load your content...
Please wait while we load your content...