A novel approach to the synthesis of bulk and supported β-Mo2C using dimethyl ether as a carbon source†
Abstract
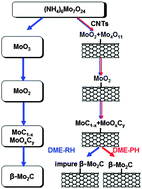
In view of the fact that H2 was necessarily added into gas-state carbon sources in order to remove the surface carbon barrier for the complete carburization of Mo oxides to form Mo carbides, in this paper we proposed a simple synthetic route to overcome this issue. The current approach used a new carbon source, dimethyl ether (DME), as feed gas without H2 addition and employed a pre-heating (PH) method before introducing DME into the reactor. With this novel approach we successfully prepared bulk and CNT supported molybdenum carbides. Note that the removal of carbon deposit by H2 from DME thermal decomposition and PH-induced surface reconstruction can promote the carburization process. The formation mechanism of Mo carbides was proposed using X-ray diffraction (XRD) and rapid heating reaction mass spectrometry (RH-MS) characterizations. The complete synthesis process involved reduction–carburization of Mo precursors by DME and their thermal decomposition product CH4, via the pathway of MoO3/Mo4O11 → MoO2 → MoOxCy/MoC1−x → β-Mo2C. The composition of gas-phase products was predominantly H2, CO and CH4 with a very small amount of CO2 and (or almost no) H2O.

 Please wait while we load your content...
Please wait while we load your content...