Zinc amidoisophthalate complexes and their catalytic application in the diastereoselective Henry reaction†
Abstract
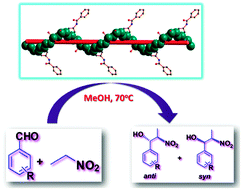
Reactions of 5-propionamidoisophthalic acid (H2L1) and 5-benzamidoisophthalic acid (H2L2) with Zn(NO3)2·6H2O under different hydrothermal conditions lead to two pairs of Zn(II) compounds, viz. the mononuclear [Zn(L1)(H2O)3]·H2O (1) and [Zn(L1)(H2O)3]·3H2O (2), and the 1D polymers [Zn(L2)(H2O)2]n (3) and [{Zn(L2)(H2O)2}·2H2O]n (4). They crystallize in orthorhombic [Pbca (1) and P212121 (3)] or triclinic (P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) , 2 and 4) systems, and their formation depends on the particular reaction conditions. Compounds 1 and 2, as well as 3 and 4, are solvatomorphs, the latter presenting different 1D metal–organic polymeric chains. All of them act as heterogeneous catalysts for the diastereoselective nitroaldol (Henry) reaction of different aldehydes with nitroalkanes and can be recycled without losing activity.
, 2 and 4) systems, and their formation depends on the particular reaction conditions. Compounds 1 and 2, as well as 3 and 4, are solvatomorphs, the latter presenting different 1D metal–organic polymeric chains. All of them act as heterogeneous catalysts for the diastereoselective nitroaldol (Henry) reaction of different aldehydes with nitroalkanes and can be recycled without losing activity.


 Please wait while we load your content...
Please wait while we load your content...