Efficient synthesis of some new antiproliferative N-fused indoles and isoquinolines via 1,3-dipolar cycloaddition reaction in an ionic liquid†
Abstract
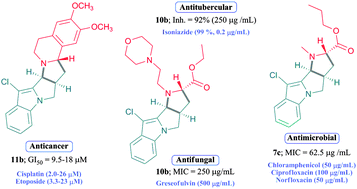
Syntheses of some new pyrrolo-fused pyrrolo[1,2-a] indole derivatives have been achieved by combining N-allyl-indole-2-carbaldehyde with a variety of N-alkyl-glycine esters as well as tetrahydroisoquinolines in an ionic liquid, triethylammonium acetate (TEAA), a recyclable reaction medium, via intramolecular [3+2] cycloaddition reaction. This new method is highly efficient, and the ionic liquid employed is recyclable. The stereochemistry of all the compounds was confirmed by 2D NMR NOESY and in some cases single crystal X-ray diffraction data. The in vitro screening of all new candidates against various bacterial strains and representative human solid tumor cell lines, A549 (lung), HeLa (cervix), SW1573 (lung), T-47D (breast) and WiDr (colon), revealed that many of them have good antibacterial, antifungal and antitubercular and antiproliferative activities.

 Please wait while we load your content...
Please wait while we load your content...