Photophysical properties, aggregation-induced fluorescence in nanoaggregates and cell imaging of 2,5-bisaryl 1,3,4-oxadiazoles†
Abstract
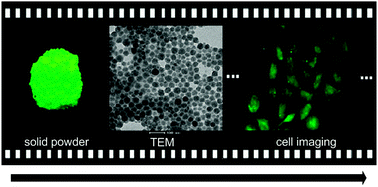
Dye-loaded nanoparticles encapsulating four different aggregation-induced emission (AIE) and intramolecular charge transfer (ICT) active chromophores, which had different numbers of carbazole-containing triphenylamine moieties as donors and one 1,3,4-oxadiazole moiety as acceptor, were investigated and reported. Dye-doped amino-group-functionalized silica nanoparticles (Si-NPs), with diameter ∼30 nm, maintained the AIE characterized photophysical properties of the chromophores with high quantum yield up to 0.35 for oxa-(BCPA)1-doped Si-NPs, and the fluorescence emission peaks ranged from 476 nm to 513 nm for the four nanoparticles. Moreover, the dye-doped Si-NPs successfully overcome the challenges of the poor biocompatibility of AIE materials and could be efficiently dispersed in water, providing an important opportunity to further explore their bioapplications by conjugating the NPs with various biomolecules. The dye-loaded BSA-nanoparticles (BSA-NPs) were applied to cell imaging, which showed a better uptake by HeLa cells compared to pure dye NPs, which suggests its promising application for biosensors such as cancer cell detection.

 Please wait while we load your content...
Please wait while we load your content...