An NMR study on the gelation of N,N′-bis(4-N-alkylo-xybenzoyl) hydrazine (4Dn) in two aromatic solvents†
Abstract
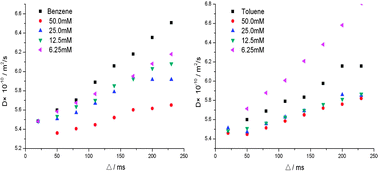
Organogels that are self-assembled from simple gelators are an interesting class of nano- and mesoscale soft matter in terms of simplicity and functionality. Investigating the precise roles of the organic solvents and their effects on stabilization of the formed organogel is an important topic for the development of low-molecular weight gelators. We found that thermal gel properties of N,N′-bis(4-N-alkylo-xybenzoyl) hydrazine (4D16) can be correlated with the Kamlet–Taft parameter gaining a detailed understanding of the solvent’s role in gelation. Benzene and toluene are similar aromatic solvents, but the 4D16 gels formed from these two solvents show a large variety of physical characteristics. We studied the non-covalent force, association constant, and thermodynamic parameters of the 4D16 aggregation process in these two aromatic solvents using NMR and explained why gelator 4D16 in toluene had a lower critical gelation concentration (CGC). We select 4D7, which has a higher CGC and its microstructures are similar to 4D16 in these two solvents, to compare differences in solvents and find that gelator–solvent interaction can break up toluene oligomers, whereas this interaction does not disrupt benzene oligomers. The motion of the gelator is restricted due to the defect size provided by the vicinity of the methyl groups in toluene oligomers, thus making gelator–gelator interaction favorable.

 Please wait while we load your content...
Please wait while we load your content...