Synthesis, structures and magnetism of heterodinuclear Ni–Ln complexes: field-induced single-molecule magnet behavior in the dysprosium analogue†
Abstract
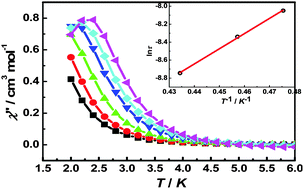
Using the hexafluoroacetylacetonato (hfac), azide anion and compartmental Schiff-base ligand H2valpn (H2valpn = 1,3-propanediylbis(2-iminomethylene-6-methoxy-phenol), three isomorphous Ni–Ln heterodinuclear complexes, [Ni(N3)(H2O)(valpn)Gd(hfac)2(H2O)]·H2O (1), [Ni(N3)(H2O)(valpn)Tb(hfac)2(H2O)]·H2O (2) and [Ni(N3)(H2O)(valpn)Dy(hfac)2(H2O)]·H2O (3), were synthesized. Structural analyses reveal that the Ni(II) and Ln(III) ions are doubly bridged by two phenoxo oxygen atoms of the valpn ligand. Magnetic susceptibility measurements show ferromagnetic couplings are operative between the Ni(II) and Ln(III) ions in 1–3. Ac susceptibility measurements under a dc-applied field of 1000 Oe disclose that the Dy derivative exhibits field-induced single-molecule magnet behavior with an effective energy barrier of 16.9 K for reversal of the magnetization.

 Please wait while we load your content...
Please wait while we load your content...