A palladium salen complex: an efficient catalyst for the Sonogashira reaction at room temperature†
Abstract
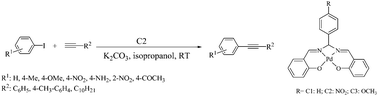
A tetradentate Schiff base derived palladium complex has been developed as a catalyst for the room temperature Sonogashira reaction under copper free conditions. The main catalytic species, a Pd-complex was derived from Schiff base ligand N,N′-bis(salicylidene)-arylmethanediamine and Pd(OAc)2. Electron rich, electron deficient and sterically hindered aryl iodides underwent smooth coupling to afford good to excellent yield of diaryl alkynes in isopropanol at room temperature. The protocol is also suitable for aliphatic alkynes.

 Please wait while we load your content...
Please wait while we load your content...