Synthesis, characterization and crystal structures of mono and dinuclear macrocyclic cobalt(ii) complexes with a new tetraaza m-xylyl-based macrocyclic ligand†
Abstract
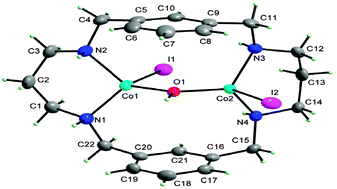
A new tetraaza macrocycle bearing two xylyl spacers was synthesized as a potential binucleating ligand (L) and the crystal structure of its tetrachloride salt was determined by single crystal X-ray diffraction analysis. Corresponding Co(II) complexes were isolated as both mono- and dinuclear complexes of [Co(H2L)Cl2]Cl2, [Co2LBr2OH]Br and [Co2LI2OH]I, and characterized using different physico-chemical techniques. The single crystal X-ray diffraction technique for [Co(H2L)Cl2]Cl2 and [Co2LI2OH]I indicates that the Co ions adopt the geometry of a distorted tetrahedron in a tetracoordinated environment. The two metal centers in the [Co2LI2OH]I complex are bridged by a hydroxyl group with Co⋯Co distance of 3.501(1) Å. The bromo- and iodo-complexes show antiferromagnetic behavior which can be described by spin only Hamiltonian while spin–orbital coupling for tetrahedral coordinating cobalt ions is very small.

 Please wait while we load your content...
Please wait while we load your content...