Effect of stacking mode on the mechanofluorochromic properties of 3-aryl-2-cyano acrylamide derivatives†
Abstract
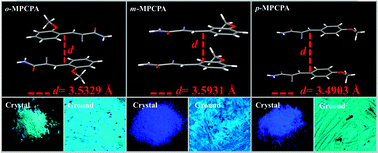
Three structurally simple 3-aryl-2-cyano acrylamide derivatives, 2-cyano-3-(2-methoxyphenyl)-2-propenamide (1), 2-cyano-3-(3-methoxyphenyl)-2-propenamide (2) and 2-cyano-3-(4-methoxyphenyl)-2-propenamide (3) were synthesized. They exhibited different optical properties due to their distinct face-to-face stacking mode. The as-prepared crystals of 1 exhibited green luminescence and the emission peak did not change after grinding treatment. However, the emission peaks of 2 (Φf = 12%) and 3 (Φf = 16%) exhibited an obvious red-shift upon grinding, and their corresponding quantum yields decreased to 8% and 10%, respectively. Differential scanning calorimetry and powder X-ray diffractometry data indicated that the optical properties of 2 and 3 could be attributed to the transformation from the crystalline phase to the amorphous phase. X-ray crystal structures, infrared spectroscopy and data from fluorescence lifetime experiments further validated the relationship between fluorescence switching, stacking mode and molecular interactions.

 Please wait while we load your content...
Please wait while we load your content...