Mechanism and application of halogen bond induced fluorescence enhancement and iodine molecule cleavage in solution†
Abstract
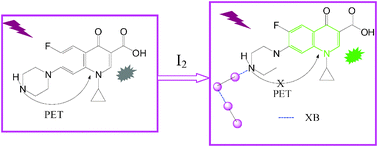
In this paper, a strong halogen bond (XB) donor (iodine) and photoinduced electron transfer (PET) molecule (ciprofloxacin, Cip) were selected with the objective to investigate halogen bonding under weakly alkaline conditions. A series of experimental characterization techniques was employed to elucidate the interaction mechanism of the XB, in combination with theoretical calculations. It is found that new UV-Vis absorption peaks and the fluorescence enhancement with the mixing of Cip and iodine are attributed to the disruption of the PET charge separation process through the halogen bonding interaction. The 2 : 1 stoichiometry of the XB complex (I2 : Cip) was attested using a modified Benesi–Hildebrand method. 1H NMR spectra showed that the iodine molecule can interact with three nitrogen atoms of Cip to form three XBs. FT-IR spectra indicated that the nitrogen atom of the imino group is the preferential interaction site of the XB. Notably, direct analysis in real time-mass spectrometry (DART-MS) gave a distinct quasi-molecular ion of the supramolecular complex (Cip + I) in solution. Meanwhile, density functional theory (DFT), taking into account the dispersion energy, revealed that the formation of an I⋯N XB not only disrupts the PET charge separation process of Cip to enhance fluorescence but also induces the cleavage of an iodine molecule (I–I) to produce a triiodine anion (I3−) XB. This explained why I3− was observed in UV-Vis and DART-MS as well as in the crystal, and how the fourth iodine atom involved in the self-assembly of the XB existed stably. Moreover, a developed optosensor based on halogen bonding has been successfully used to analyze commercial Cip·HCl capsules, suggesting the potential applicability of halogen bonding in real pharmaceutical analyses.

 Please wait while we load your content...
Please wait while we load your content...