C5-curcuminoid-4-aminoquinoline based molecular hybrids: design, synthesis and mechanistic investigation of anticancer activity†
Abstract
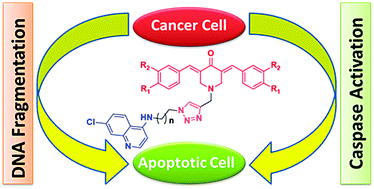
The privileged scaffolds of curcumin and 4-aminoquinolines are extensively used in the design and synthesis of biodynamic agents having remarkable efficacy against diseases like cancer and malaria. Therefore, we anticipated that covalent hybridization of these two pharmacophores via the triazole linker may lead to molecules with better anticancer activity. The synthesized hybrid compounds were tested for their anti-cancer activity on 60 human cancer cell lines, which represent diverse histologies. Our study has identified a set of these hybrids that showed excellent growth inhibition at nano-molar concentrations. The mechanistic investigations through a series of assays showed apoptotic induction as a cause for their displayed anticancer activity.

 Please wait while we load your content...
Please wait while we load your content...