Iron-dependent turnover of IRP-1/c-aconitase in kidney cells†
Abstract
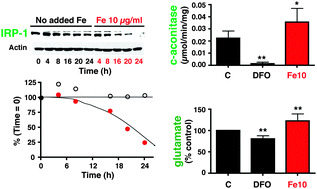
The kidney plays an important role in iron homeostasis and actively reabsorbs citrate. The bifunctional iron-regulatory protein IRP-1 potentially regulates iron trafficking and participates in citrate metabolism as a cytosolic (c-) aconitase. We investigated the role of cellular iron status in determining the expression and dynamics of IRP-1 in two renal cell types, with the aim of identifying a role of the protein in cellular ROS levels, citrate metabolism and glutamate production. The effects of iron supplementation and chelation on IRP-1 protein and mRNA levels and protein turnover were compared in cultured primary rat mesangial cells and a porcine renal tubule cell line (LLC-PK1). Levels of ROS were measured in both cell types, and c-aconitase activity, glutamate, and glutathione were measured in LLC-PK1 cells, with and without IRP-1 silencing and in glutamine-supplemented or nominally glutamine-free medium. Iron supplementation decreased IRP-1 levels (e.g., approx. 40% in mesangial cells treated with 10 μg ml−1 iron for 16 h) and increased ubiquitinated IRP-1 levels in both cells types, with iron chelation having the opposite effect. Although iron increased ROS levels (three-fold with 20 μg ml−1 iron in mesangial cells and more modestly by about 30% with 50 μg ml−1 in LLC-PK1 cells, both after 24 h), protein degradation was not ROS-dependent. In LLC-PK1 cells, 10 μg ml−1 iron (24 h) increased both aconitase activity (30%) and secreted glutamate levels (65%). Silencing did not remove the glutamate response to iron but decreased the c-aconitase activity of the residual protein independent of iron loading (37% and 46% of control levels, without and with iron treatment, respectively). However, in glutamine-free medium, glutamate was still increased by iron, even in IRP-1-silenced cells, and did not correspond to c-aconitase. Silencing decreased the amount of ferritin measured in response to iron loading, decreased the affect of iron on total glutathione by 48%, and increased the response of ROS to iron loading by 38%. We conclude that iron increases turnover of IRP-1 in kidney cells, while increasing aconitase activity, suggesting that the apoprotein (aconitase-inactive) form is not exclusively responsible for turnover. Iron increases glutamate levels in tubule epithelial cells, but this appears to be independent of c-aconitase activity or the availability of extracellular glutamine. IRP-1 protein levels are not regulated by ROS, but IRP-1-dependent ferritin expression may decrease ROS and increase total glutathione levels, suggesting that ferritin levels are more important than citrate metabolism in protecting renal cells against iron.

 Please wait while we load your content...
Please wait while we load your content...