Synthesis and anti-cancer screening of novel heterocyclic-(2H)-1,2,3-triazoles as potential anti-cancer agents
Abstract
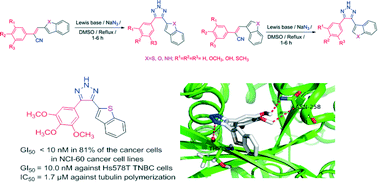
trans-Cyanocombretastatin A-4 (trans-CA-4) analogues have been structurally modified to afford their more stable CA-4-(2H)-1,2,3-triazole analogues. Fifteen novel, stable 4-heteroaryl-5-aryl-(2H)-1,2,3-triazole CA-4 analogues (8a–i, 9 and 11a–e) were evaluated for anti-cancer activity against a panel of 60 human cancer cell lines. These analogues displayed potent cytotoxic activity against both hematological and solid tumor cell lines with GI50 values in the low nanomolar range. The most potent compound, 8a, was a benzothiophen-2-yl analogue that incorporated a 3,4,5-trimethoxyphenyl moiety connected to the (2H)-1,2,3-triazole ring system. Compound 8a exhibited GI50 values of <10 nM against 80% of the cancer cell lines in the panel. Three triazole analogues, 8a, 8b and 8g, showed particularly potent growth inhibition against the triple negative Hs578T breast cancer cell line with GI50 values of 10.3 nM, 66.5 nM and 20.3 nM, respectively. Molecular docking studies suggest that these compounds bind to the same hydrophobic pocket at the interface of α- and β-tubulin that is occupied by colchicine and cis-CA-4, and are stabilized by Van der Waals' interactions with surrounding amino acid residues. Compound 8a was found to inhibit tubulin polymerization in vitro with an IC50 value of 1.7 μM. The potent cytotoxicity of these novel compounds and their inhibition of tubulin dynamics make these triazole analogues promising candidates for development as anti-cancer drugs.

 Please wait while we load your content...
Please wait while we load your content...