SDRL: a sequence-dependent protein side-chain rotamer library†
Abstract
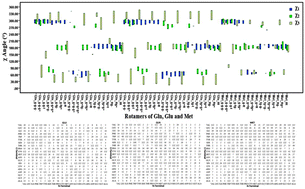
Since the introduction of the first protein side-chain rotamer library (RL) almost half a century ago, RLs have been components of many programs and algorithms in structural bioinformatics. Based on the dependence of side-chain dihedral angles on the local backbone, three types of RLs have been identified: backbone-independent, secondary-structure-dependent and backbone-dependent. In all previous studies, the effect of sequence specificity on side-chain conformational preferences was neglected. In the effort to develop a new class of RLs, we considered that the side-chain conformation of the central residue in each triplet on a protein backbone depends on the sequence of the triplet; therefore, we developed a sequence-dependent rotamer library (SDRL). To accomplish this, 400 possible triplet sequences for 18 natural amino acids as the central residue, which corresponds to 7200 triplet sequences in total, were considered. Searching the set of 11 546 selected PDB entries for the 7200 triplet sequences resulted in 2 364 541 instances occurring for 18 amino acids. Our results show that Leu and Val experience minimal impact from the adjacent residues in adopting side-chain conformations. Cys, Ile, Trp, His, Asp, Met, Glu, Gln, Arg and Lys, on the other hand, adopt their side-chain conformations mostly based on the adjacent residues on the backbone. The remaining residue types were moderately dependent on the adjacent residues. Using the new library, side-chain repacking algorithms can find preferred conformations of each residue more easily than with other backbone-independent RLs.
- This article is part of the themed collection: Chemical Biology in Molecular BioSystems

 Please wait while we load your content...
Please wait while we load your content...