Cell pinball: phenomenon and mechanism of inertia-like cell motion in a microfluidic channel†
Abstract
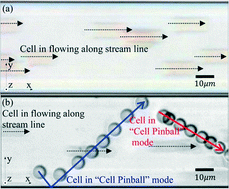
An unexpected phenomenon of red blood cells bouncing back and forth between the walls inside a microfluidic channel was observed during experiments, and is presented as “Cell Pinball” in this paper. In general, cells in a microfluidic environment are supposed to move along the streamlines parallel to the channel walls when the Reynolds number is small, and the inertia of the cells becomes negligible. However, the cell pinball presented in this paper does not only move along the streamlines but also moves across the channel with the velocity component perpendicular to the streamlines while the Reynolds number is only 0.74. Furthermore, the motion in the direction perpendicular to the streamlines reverses when the cell pinball hits a wall as it “bounces” at the wall. This phenomenon caught our attention and is investigated with both microbead visualization and confocal microscopy. Consistent patterns of rotation with respect to the direction of motion are observed. A kinematic model is proposed to interpret the phenomenon, and it is believed that the phenomenon is caused by the separation of the centroid of the cell and the contact point. The model successfully interprets the features of cell pinball, and the estimated separation between the centroid and the contact point is presented.

 Please wait while we load your content...
Please wait while we load your content...