Mechanisms of integrin and filamin binding and their interplay with talin during early focal adhesion formation†
Abstract
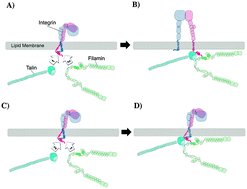
Filamin plays a key role in cellular biomechanics as an actin cross-linker and as a versatile focal adhesion binding partner. It binds directly to integrins, a family of mechanosensitive transmembrane receptors that mediate attachment to several extracellular ligands such as fibronectin, collagen, and laminin. Filamin binds β-integrin at its cytoplasmic tail, competing with talin, a major integrin activator that plays a chief role in cell adhesion. Herein, we develop molecular dynamics models to study the mechanism of early binding of αIIbβ3 integrin with filamin A (FLNa). Our models predict three important electrostatic interactions and one stabilizing hydrophobic interaction that mediate binding between filamin and integrin. In its native conformation, filamin's integrin binding site is auto-inhibited. Our models help shed light on the role of integrin binding on regulating filamin activation. Finally, the effect of talin on the filamin–integrin interaction is explored and possible scenarios of the interplay among these molecules are examined.
- This article is part of the themed collection: Mechanobiology

 Please wait while we load your content...
Please wait while we load your content...