Sustainable allylation of organosolv lignin with diallyl carbonate and detailed structural characterization of modified lignin†
Abstract
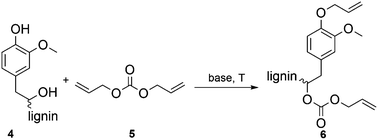
The allylation of European beech wood organosolv lignin (OL) with diallyl carbonate (DAC) in the presence of base catalysts is shown to be an efficient, non-toxic, and sustainable modification technique. Comparative studies of different bases and temperatures were performed to optimize the solvent-free allylation of OL. Up to 90% of the aromatic and aliphatic hydroxyl groups in OL were converted to the respective allyl ethers or allyl carbonates using tetrabutylammonium bromide (TBAB) as recyclable base. The demonstrated strategy allows the introduction of functional groups into the structure of lignin, which will enable further modification. Detailed structural analytics by 31P, 1H, and 13C NMR, as well as IR spectroscopy and detailed mass analysis using SEC–ESI–MS verified the functionalization and delivered insights into the structure of lignin.

 Please wait while we load your content...
Please wait while we load your content...