Chemo-enzymatic synthesis of key intermediates (S)-γ-hydroxymethyl-α,β-butenolide and (S)-γ-hydroxymethyl-γ-butyrolactone via lipase-mediated Baeyer–Villiger oxidation of levoglucosenone†
Abstract
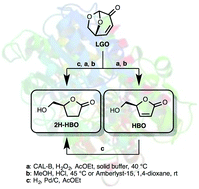
Levoglucosenone (LGO), a valuable chiral platform chemical that can be efficiently produced from catalytic fast pyrolysis of cellulose, has been efficiently converted into optically pure (S)-γ-hydroxymethyl-α,β-butenolide (HBO) using a two-step sequence involving a lipase-mediated Baeyer–Villiger oxidation and an acid hydrolysis. In the same fashion, (S)-γ-hydroxymethyl-γ-butyrolactone (2H-HBO) was successfully obtained through a three-step sequence (Baeyer–Villiger, palladium-catalysed hydrogenation and acid hydrolysis). The use of solid buffers in the lipase-mediated Baeyer–Villiger oxidation has proved beneficial in two ways: not only the reaction time and the enzymatic load were both reduced four-fold (from 8 to 2 hours and 464 to 113 U mmol−1) to reach conversions ≥83%, but solid buffers also prevented lipase from denaturation, thus preserving its enzymatic activity and allowing its use for further oxidation cycles.

 Please wait while we load your content...
Please wait while we load your content...