Efficient catalytic hydrotreatment of Kraft lignin to alkylphenolics using supported NiW and NiMo catalysts in supercritical methanol†
Abstract
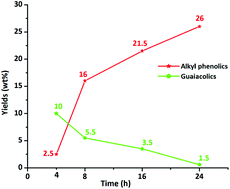
Efficient catalytic hydrotreatment of Kraft lignin to yield aromatic monomers was demonstrated in supercritical methanol using a variety of NiW and NiMo catalysts on acidic, basic and neutral supports. It was found that NiW catalysts on neutral or basic supports are highly suitable for depolymerization of Kraft lignin to methanol soluble organics in high yields at 320 °C and 35 bar H2 pressure. An extensive analysis of the product mixtures was carried out using GC-MS-FID, GC × GC-FID, 2D HSQC NMR, GPC and elemental analysis, and several techniques were used for the characterization of the prepared catalysts in order to determine the acidity and basicity of the support and morphological changes after the catalytic reaction. The best results were obtained with sulphided NiW catalysts supported on activated carbon. Efficient depolymerization of Kraft lignin and a total 28 wt% monomer yield was obtained within 8 h and 76% of the products were alkylphenolics and guaiacolics. Over prolonged reaction times, the total monomer yield reached 35 wt%, containing up to 26 wt% alkylphenolics. During catalytic processing, deoxygenation was the most prevalent reaction and, importantly, no competing aromatic ring hydrogenation or undesired repolymerization to insoluble char was observed. The catalytic system described here represents a highly efficient and selective method for the production of alkylphenolics and guaiacolics from Kraft lignin.
- This article is part of the themed collection: Lignin chemistry and valorisation

 Please wait while we load your content...
Please wait while we load your content...