Exploring glutathione lyases as biocatalysts: paving the way for enzymatic lignin depolymerization and future stereoselective applications†
Abstract
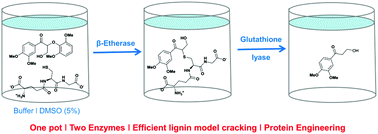
Glutathione-dependent β-etherases and glutathione lyases are key-enzymes for the biocatalytic depolymerization of lignin. In the first step, the nucleophilic attack of glutathione to the common β-O-4-aryl-ether motif in lignin is catalyzed by β-etherases and afterwards the glutathione is removed again by the action of glutathione lyases. Given their potential impact for lignin valorization, in this paper novel glutathione lyases are reported and biocatalytically characterized based on lignin model compounds. As a result, an enzyme exhibiting increased thermostability and lowered enantioselectivity – key features for implementation of glutathione lyases in enzymatic lignin depolymerization processes – was identified. Furthermore, first mutational studies of these enzymes revealed the possibility to further alter the activity as well as enantioselectivity of glutathione lyases by means of protein engineering. From a practical perspective, one-pot multi-step processes combining β-etherases and glutathione lyases are successfully set-up, giving hints on the potential that the implementation of these biocatalysts may bring for biorefinery purposes.
- This article is part of the themed collection: Lignin chemistry and valorisation

 Please wait while we load your content...
Please wait while we load your content...