Solid state synthesis of nano-sized AlH3 and its dehydriding behaviour†
Abstract
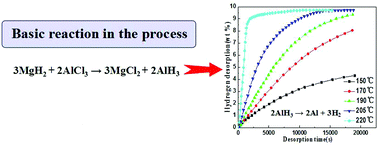
Aluminum hydride (AlH3) has a high gravimetric hydrogen capacity (10.1 wt%) and has attracted considerable attention due to its potential application for hydrogen storage. Up to now, almost all the routes developed for the synthesis of AlH3 are energy-consuming and economically impractical for mass production. In this study, a cost effective route of solid state reactive milling was proposed to synthesize AlH3 using aluminum chloride and cheap metal hydrides as starting reagents, with the LiH/AlCl3, MgH2/AlCl3, and CaH2/AlCl3 reaction systems being experimentally investigated. The reaction progress and products during reactive milling were characterized by XRD and 27Al NMR, and the morphology as well as the microstructure of the as-milled samples by SEM and TEM, respectively. It was found that nano-sized γ-AlH3 could be synthesized by reactive milling with commercial AlCl3 and nanocrystalline MgH2 as reagents. Based on the XRD and NMR analyses as well as the TEM observation, the average size of the γ-AlH3 phase in the as-synthesized γ-AlH3/MgCl2 nanocomposite was estimated to be about 8.5 nm. By an isothermal dehydrogenation test, the as-synthesized γ-AlH3 was found to have a quite high hydrogen desorption capacity and fast kinetics, with a hydrogen desorption amount of about 9.71 wt% within 9080 s at 220 °C.

 Please wait while we load your content...
Please wait while we load your content...