An enzymatic route to a benzocarbazole framework using bacterial CotA laccase†
Abstract
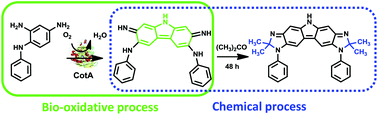
The CotA laccase-catalysed oxidation of the meta,para-disubstituted arylamine 2,4-diaminophenyldiamine delivers, under mild reaction conditions, a benzocarbazole derivative (1) (74% yield), a key structural motif of a diverse range of applications. This work extends the scope of aromatic frameworks obtained using these enzymes and represents a new efficient and clean method to construct in one step C–C and C–N bonds.

 Please wait while we load your content...
Please wait while we load your content...