One-pot triangular chemoenzymatic cascades for the syntheses of chiral alkaloids from dopamine†
Abstract
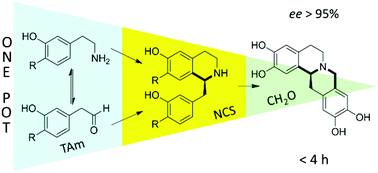
We describe novel chemoenzymatic routes to (S)-benzylisoquinoline and (S)-tetrahydroprotoberberine alkaloids using the enzymes transaminase (TAm) and norcoclaurine synthase (NCS) in a one-pot, one-substrate ‘triangular’ cascade. Employment of up to two C–C bond forming steps allows for the rapid generation of molecular complexity under mild conditions.
- This article is part of the themed collection: 2015 most accessed Green Chemistry articles


 Please wait while we load your content...
Please wait while we load your content...