Synergistic effects of Ni and acid sites for hydrogenation and C–O bond cleavage of substituted phenols
Abstract
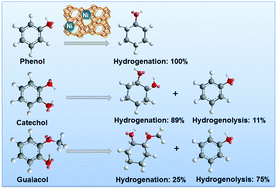
The cleavage of C–O bonds in phenol, catechol, and guaiacol has been explored with mono- and dual-functional catalysts containing Ni and/or HZSM-5 in the aqueous phase. The aromatic ring of phenol is hydrogenated in the first step, and the C–O bond of the resulting cyclohexanol is dehydrated in sequence. The initial turnover frequency (TOF) of phenol hydrodeoxygenation increases in parallel with the acid site concentration irrespective of the concentration of the accessible surface Ni atoms. For catechol and guaiacol conversion, Ni catalyzes the hydrogenolysis of the C–O bonds in addition to arene hydrogenation. For catechol, the hydrogenation of the aromatic ring and the hydrogenolysis of the phenolic –OH group occur in parallel with a ratio of 8 : 1. The saturated cyclohexane-1,2-diol can be further dehydrated over HZSM-5 or hydrogenolyzed on Ni to complete hydrodeoxygenation. Guaiacol undergoes primarily hydrogenolysis (75%) to phenol via demethoxylation, and the hydrogenation route accounts for only 25%. This is attributed to the steric effects arising from the adjacent sp3 hybrid O–CH3 group. 2-Methoxycyclohexanol (from guaiacol hydrogenation) reacts further either via hydrogenolysis by Ni to cyclohexanol or via acid catalyzed demethoxylation and rearrangement steps followed by the subsequent hydrogenation of the intermediately formed olefins. On Ni/HZSM-5, the hydrodeoxygenation activities are much higher for the phenolic monomers than for their respective saturated analogues, pointing to the importance of sp2 orbitals. The presence of proximal acid sites increases the activities of Ni in the presence of H2 by a synergistic action.

 Please wait while we load your content...
Please wait while we load your content...