Colloidal stability of (functionalised) fullerenes in the presence of dissolved organic carbon and electrolytes
Abstract
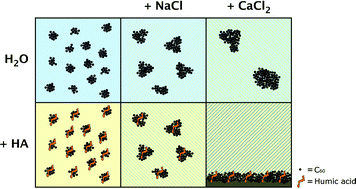
Carbon-based nanoparticles such as fullerenes have been widely applied in personal care products, drug delivery systems, and solar cells. The properties of nanoparticles have been increasingly studied because of their applications and their potential risks to the environment and human health. Many studies have focused on the environmental fate and properties of C60. However, there is limited information available on the environmental properties of functionalised fullerenes. This study focuses on the colloidal stability of two fullerenes (C60 and [6,6]-diphenyl-C62-bis(butyric acid methyl ester)) in water in the presence of dissolved organic carbon (DOC) and different electrolytes (NaCl and CaCl2). Suspended fullerene concentrations were determined with high resolution Orbitrap mass spectrometry. The size was determined by multi-angle light scattering, dynamic light scattering and for the first time by flow cytometry. The suspended concentrations of the fullerenes were stabilised by low concentrations of DOC (2 mg C per L) in the presence of NaCl. However, sedimentation of DOC occurred at low concentrations of CaCl2 (>~2 mM) which caused removal of (functionalised) fullerenes. The results show that (functionalised) fullerenes can be rapidly removed in natural aqueous systems in the presence of low concentrations of DOC and multivalent inorganic electrolytes.

 Please wait while we load your content...
Please wait while we load your content...