Selective, high-temperature permeation of nitrogen oxides using a supported molten salt membrane†
Abstract
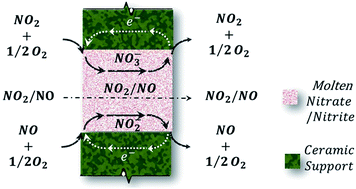
Molten nitrate/ceramic membranes of KNO3/Al2O3 and KNO3/La0.6Sr0.4Co0.2Fe0.8O3−δ (LSCF) were designed and successfully operated for the selective permeation of nitrogen oxides. With the presence of oxygen on the feed side of the membrane, the KNO3/Al2O3 membrane showed no measurable oxygen permeation while the KNO3/LSCF membrane exhibited nitrogen dioxide and oxygen co-permeation. For the KNO3/LSCF membrane, a permeance of up to 6.9 × 10−8 mol m−2 s−1 Pa−1 was achieved while the nitrogen dioxide to oxygen permeate ratio appeared to be approximately constant with a value of around unity.

 Please wait while we load your content...
Please wait while we load your content...