Scalability and feasibility of photoelectrochemical H2 evolution: the ultimate limit of Pt nanoparticle as an HER catalyst†
Abstract
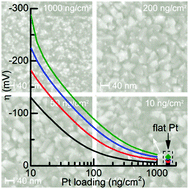
The recent surge in investigating electrocatalysts for the H2 evolution reaction is based on finding a cheap alternative to Pt. However platinum's excellent catalytic activity means very little catalyst needs to be used. The present study combines model experiments with numerical modeling to determine exactly how little catalyst is needed. Specifically we investigate ultra-low Pt loadings for use in photoelectrochemical H2 evolution using TiO2–Ti-pn+Si photocathodes. At a current density of 10 mA cm−2, we photocathodically evolve H2 at +465, +450, +350 and +270 mV vs., RHE at Pt loadings of 1000, 200, 50, and 10 ng cm−2 corresponding to HER overpotentials of η1000ng = 32 mV, η200ng = 46 mV, η50ng = 142 mV, and η10ng = 231 mV. To put this in perspective, if 30% of the world's current annual Pt production was used for H2 evolution catalysis, using a loading of 100 ng cm−2 and a current of 10 mA cm−2 would produce 1 TWaverage of H2. The photoelectrochemical data matched the modeling calculations implying that we were near the fundamental maximum in performance for our system. Furthermore modeling indicated that the overpotentials were dominated by mass transfer effects, rather than catalysis unless catalyst loadings were less than 1000 ng cm−2.


 Please wait while we load your content...
Please wait while we load your content...