Sodium intercalation chemistry in graphite†
Abstract
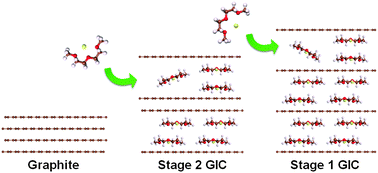
The insertion of guest species in graphite is the key feature utilized in applications ranging from energy storage and liquid purification to the synthesis of graphene. Recently, it was discovered that solvated-Na-ion intercalation can occur in graphite even though the insertion of Na ions alone is thermodynamically impossible; this phenomenon enables graphite to function as a promising anode for Na-ion batteries. In an effort to understand this unusual behavior, we investigate the solvated-Na-ion intercalation mechanism using in operando X-ray diffraction analysis, electrochemical titration, real-time optical observation, and density functional theory (DFT) calculations. The ultrafast intercalation is demonstrated in real time using millimeter-sized highly ordered pyrolytic graphite, in which instantaneous insertion of solvated-Na-ions occurs (in less than 2 s). The formation of various stagings with solvated-Na-ions in graphite is observed and precisely quantified for the first time. The atomistic configuration of the solvated-Na-ions in graphite is proposed based on the experimental results and DFT calculations. The correlation between the properties of various solvents and the Na ion co-intercalation further suggests a strategy to tune the electrochemical performance of graphite electrodes in Na rechargeable batteries.


 Please wait while we load your content...
Please wait while we load your content...