Achieving overall water splitting using titanium dioxide-based photocatalysts of different phases†
Abstract
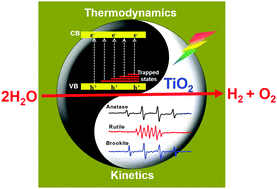
Titanium dioxide (TiO2) is regarded as the benchmark semiconductor in photocatalysis, which possesses a suitable band structure and makes the overall water splitting reaction thermodynamically possible. However, photocatalytic overall water splitting (POWS) (2H2O → 2H2 + O2) can only take place on rutile but hardly on anatase and brookite TiO2. So obtaining the POWS on TiO2-based photocatalysts has remained a long-standing challenge for over 40 years. In this work, we found that the POWS on anatase and brookite TiO2 becomes feasible under prolonged UV light irradiation. Further investigation by means of electron spin resonance spectroscopy (EPR) and transient infrared absorption–excitation energy scanning spectroscopy (TRIRA-ESS) reveals that both kinetics and thermodynamics factors contributed to unique POWS activity for different phases of TiO2. Kinetically the process of photocatalysis differs on different phases of TiO2 due to the intermediates (˙OH radical for anatase and brookite TiO2, peroxy species for rutile TiO2) that are formed. Thermodynamically there are many trapped states lying near the valence band of anatase and brookite but not for rutile TiO2, which reduce the overpotential for water oxidation. These findings develop our understanding of why some semiconductors are inactive as POWS photocatalysts despite having thermodynamically suitable band structures for the proton reduction and water oxidation reactions.
- This article is part of the themed collection: 2015 most accessed Energy & Environmental Science articles

 Please wait while we load your content...
Please wait while we load your content...