Convergent reductive depolymerization of wood lignin to isolated phenol derivatives by metal-free catalytic hydrosilylation†
Abstract
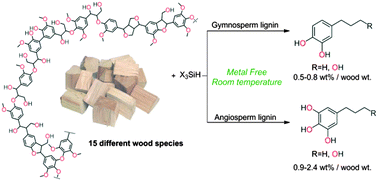
The first examples of reductive depolymerization of lignin are reported under metal-free conditions. Using hydrosilanes as reductants and B(C6F5)3 as a Lewis acid catalyst, wood lignin is efficiently converted to a narrow distribution of phenol derivatives at room temperature. A three-step methodology based on the selection of the wood species and the lignin extraction method followed by a convergent reductive depolymerization enables the production of four structurally defined aromatic compounds. The phenol products were successfully isolated in 7–24 wt% yield from lignin and 0.5–2.4 wt% yield from wood. The strategy is found to be robust and is applied to 15 different wood plants, including gymnosperm and angiosperm species. The efficiency of this novel methodology has been evaluated based on spectroscopic characterization of the lignin preparations and isolated yields of mono-aromatic products.

 Please wait while we load your content...
Please wait while we load your content...