Lithium salts for advanced lithium batteries: Li–metal, Li–O2, and Li–S
Abstract
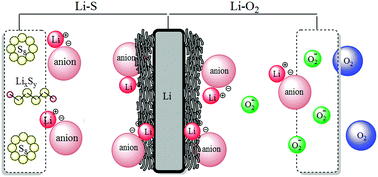
Presently lithium hexafluorophosphate (LiPF6) is the dominant Li-salt used in commercial rechargeable lithium-ion batteries (LIBs) based on a graphite anode and a 3–4 V cathode material. While LiPF6 is not the ideal Li-salt for every important electrolyte property, it has a uniquely suitable combination of properties (temperature range, passivation, conductivity, etc.) rendering it the overall best Li-salt for LIBs. However, this may not necessarily be true for other types of Li-based batteries. Indeed, next generation batteries, for example lithium–metal (Li–metal), lithium–oxygen (Li–O2), and lithium–sulfur (Li–S), require a re-evaluation of Li-salts due to the different electrochemical and chemical reactions and conditions within such cells. This review explores the critical role Li-salts play in ensuring in these batteries viability.

 Please wait while we load your content...
Please wait while we load your content...