Environmental and economic assessment of lactic acid production from glycerol using cascade bio- and chemocatalysis†
Abstract
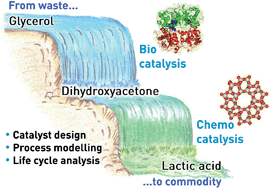
Recently, lactic acid has emerged as one of the most relevant platform molecules for the preparation of bio-chemicals. Due to the limited productivity of sugar fermentation, the dominant industrial technology practiced for its manufacture, new chemocatalytic processes are being developed in order to meet the expected demand for this intermediate. The Lewis-acid catalysed isomerisation of dihydroxyacetone has attracted particular interest. If the reaction is performed in water, lactic acid is attained directly, while if alcohol is used as the solvent, the desired product can be obtained upon subsequent hydrolysis of the alkyl lactates formed. Herein, we (i) demonstrate tin-containing MFI zeolites prepared by scalable methods as highly active, selective and recyclable catalysts able to operate in concentrated dihydroxyacetone aqueous and methanolic solutions, and (ii) reveal by life cycle analysis that a process comprising the enzymatic production of dihydroxyacetone from crude glycerol and its chemocatalytic isomerisation in methanol is advantageous for the production of lactic acid compared to glucose fermentation in terms of both sustainability and operating costs. In particular, we demonstrate that the reduced energy requirements and CO2 emissions of the cascade process originate from the valorisation of a waste feedstock and from the high performance and recyclability of the zeolite catalyst and that the economic advantage is strongly determined by the comparably low market price of glycerol. It is also shown that the bio-/chemocatalytic route remains ecologically and economically more attractive even if the purity of glycerol is as low as 38%.
- This article is part of the themed collection: Celebrating the 2017 RSC Prize and Award Winners

 Please wait while we load your content...
Please wait while we load your content...