Platinum(ii)–porphyrin as a sensitizer for visible-light driven water oxidation in neutral phosphate buffer†
Abstract
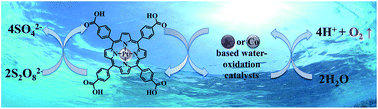
A water-soluble Pt(II)–porphyrin with a high potential for one-electron oxidation (∼1.42 V vs. NHE) proves very suitable for visible-light driven water oxidation in neutral phosphate buffer solution in combination with a variety of water oxidation catalysts (WOCs). Two homogeneous WOCs (iridium(N-heterocyclic carbene) and Co4O4–cubane complexes) and two heterogeneous WOCs (IrOx·nH2O and Co3O4 nanoparticles) were investigated, with sodium persulfate (Na2S2O8) as a sacrificial electron acceptor. Under neutral buffer conditions, the Pt(II)–porphyrin shows higher stability than the commonly used photosensitizer [Ru(bpy)3]2+, and therefore represents a good alternative photosensitizer to be used in the evaluation of light driven WOCs.

 Please wait while we load your content...
Please wait while we load your content...