Amino-ether macrocycle that forms CuII templated threaded heteroleptic complexes: a detailed selectivity, structural and theoretical investigations†
Abstract
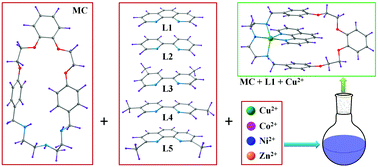
A new oxy(ether)tris(amine) macrocycle, MC has been synthesized for CuII complex formation within the cavity of the macrocyclic wheel in endotopic fashion. This complex is further reacted with the bidentate chelating ligands, 1,10-phenanthroline (L1), 2,2′-bipyridyl (L2), 4,4′-dimethyl-2,2′-bipyridyl (L3) and 5,5′-dimethyl-2,2′-bipyridyl (L4) to achieve the pseudorotaxanes PRT1–PRT4, respectively. These bis-heteroleptic complexes were characterized by the electrospray ionization mass spectrometry (ESI-MS), UV/Vis, EPR spectroscopy and Single-crystal X-ray structural analysis. Binding constants of the heteroleptic complexes were found in the range of 1.16 × 102 to 1.55 × 103 M−1 in acetonitrile. Further, the double level selectivity studies of MC using different metal ions [CoII, NiII, CuII, ZnII] and a number of simple bidentate chelating ligands shows the selective formation of PRT1 that justifies the self-sorting nature of the system. Further, substitution of axles from pseudorotaxanes PRT2–PRT4 could also be achieved by L1 with nearly 100% efficiency. To corroborate the experimental studies (comparison with the crystal geometry, importance of the π–π stacking, etc.) the geometries of the pseudorotaxanes were optimized using DFT (B3LYP) in the gas phase.
- This article is part of the themed collection: New Talent: Asia-Pacific

 Please wait while we load your content...
Please wait while we load your content...