Aqueous phase selective detection of 2,4,6-trinitrophenol using a fluorescent metal–organic framework with a pendant recognition site†
Abstract
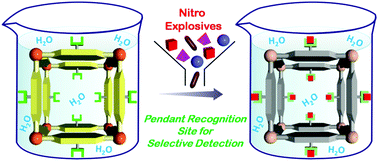
Prompt and selective detection of nitro explosives in the aqueous phase is in high demand to meet homeland security and environmental concerns. Herein we report the chemically stable porous metal organic framework UiO-68@NH2 with a pendant recognition site for selective detection of the nitro-aromatic explosive TNP in the aqueous phase. The pendant Lewis basic amine moieties are expected to selectively interact with TNP via electrostatic interactions and act as recognition sites for TNP. The MOF can detect the presence of TNP in water at a concentration as low as 0.4 ppm with a response time of a few seconds. In addition, both excitation and emission wavelengths of the MOF are in the visible region. The high selectivity was observed even in the presence of competing nitro analytes in the aqueous phase. The quenching constant for TNP was found to be 5.8 × 104 M−1 which is 23 times higher than that for TNT and for RDX, demonstrating superior and selective quenching ability. This unprecedented selectivity is ascribed to electron-transfer and energy-transfer mechanisms as well as electrostatic interactions between TNP and the MOF. An MOF-coated paper strip that we prepared demonstrated fast and selective response to TNP in water, which represents a first step towards a practical application.
- This article is part of the themed collection: New Talent: Asia-Pacific


 Please wait while we load your content...
Please wait while we load your content...