Intraprotein transmethylation via a CH3–Co(iii) species in myoglobin reconstituted with a cobalt corrinoid complex†
Abstract
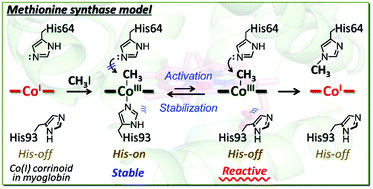
Myoglobin reconstituted with a cobalt tetradehydrocorrin derivative, rMb(Co(TDHC)), was investigated as a hybrid model to replicate the reaction catalyzed by methionine synthase. In the heme pocket, CoI(TDHC) is found to react with methyl iodide to form the methylated cobalt complex, CH3–CoIII(TDHC), although it is known that a similar nucleophilic reaction of a cobalt(I) tetradehydrocorrin complex does not proceed effectively in organic solvents. Furthermore, we observed a residue- and regio-selective transmethylation from the CH3–CoIII(TDHC) species to the Nε2 atom of the His64 imidazole ring in myoglobin at 25 °C over a period of 48 h. These findings indicate that the protein matrix promotes the model reaction of methionine synthase via the methylated cobalt complex. A theoretical calculation provides support for a plausible reaction mechanism wherein the axial histidine ligation stabilizes the methylated cobalt complex and subsequent histidine-flipping induces the transmethylation via heterolytic cleavage of the Co–CH3 bond in the hybrid model.

 Please wait while we load your content...
Please wait while we load your content...