Identification, structural characterization and transformations of the high-temperature Zn9−δSb7 phase in the Zn–Sb system
Abstract
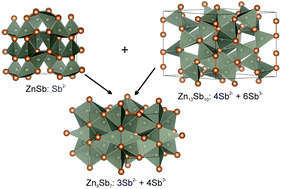
The Zn9−δSb7 phase has been identified via high-temperature powder diffraction studies. Zn9−δSb7 adopts two modifications: an α form stable between 514 °C and 539 °C and a Zn-poorer β form stable from 539 °C till its melting temperature of 581 °C. The Zn9−δSb7 structure was solved from the powder data using the simulated annealing approach. Both modifications adopt the same hexagonal structure (P6/mmm) but with slightly different lattice parameters. The α-to-β transformation is abrupt and first-order in nature. The Zn atoms occupy the tetrahedral holes created by Sb atoms. The ideal Zn9Sb7 composition can be explained by its tendency to adopt a charge balance configuration. Out of 7 Sb atoms, 3 Sb atoms form dimers (Sb2− ions) and 4 Sb atoms are isolated (Sb3− ions), which require 9 Zn2+ cations for charge neutrality.

 Please wait while we load your content...
Please wait while we load your content...