Which one is faster? A kinetic investigation of Pd and Ni catalyzed Negishi-type oxidative coupling reactions†
Abstract
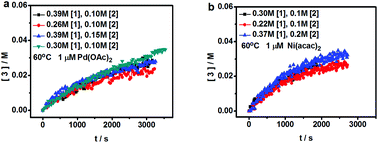
The difference between Pd and Ni has been investigated based on the Negishi-type oxidative coupling reactions in which reductive elimination was proved to be the rate determining step. Although DFT calculations illustrate that the Pd catalyzed reaction should be faster than the Ni catalyzed reaction under these conditions, kinetic experiments indicate that the reaction rate of Pd and Ni is dependent on the concentration of the catalyst precursor. The Pd catalyzed reaction is faster than the Ni catalyzed reaction only when the precursor concentration is as low as 1 × 10−7 M.


 Please wait while we load your content...
Please wait while we load your content...