Transition metal-mediated donor–acceptor coordination of low-oxidation state Group 14 element halides†
Abstract
The reactivity of tungsten carbonyl adducts of Group 14 element (Ge, Sn and Pb) dihalides towards the metal-based donors (η5-C5H5)Rh(PMe2Ph)2 and Pt(PCy3)2 was examined. When (η5-C5H5)Rh(PMe2Ph)2 was treated with the Lewis acid supported Ge(II) complex, THF·GeCl2·W(CO)5, cyclopentadienyl ring activation occurred, whereas the analogous Lewis acidic units SnCl2·W(CO)5 and PbCl2 form direct adducts with the Rh complex to yield Rh–Sn and Rh–Pb dative bonds. Attempts to prepare metal coordinated element(II) hydrides by adding hydride sources to the above mentioned rhodium–E(II) halide complexes were unsuccessful; in each case insoluble products were formed along with regeneration of free (η5-C5H5)Rh(PMe2Ph)2. In a parallel study, ECl2·W(CO)5 (E = Ge or Sn) groups were shown to participate in E–Cl oxidation addition chemistry with (Cy3P)2Pt to give the formal Pt(II) complexes ClPt(PCy3)2ECl·W(CO)5.
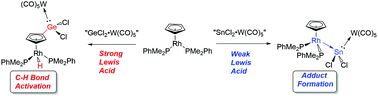
- This article is part of the themed collection: Main Group Transformations

 Please wait while we load your content...
Please wait while we load your content...